Let’s fit some models of continuous character evolution. First, we will learn how to do some tests of “phylogenetic signal,” a very common test especially for ecological analyses. Then we will learn how to fit a series of evolutionary models for continuous characters.
library(geiger)
## Loading required package: ape
library(picante)
## Loading required package: vegan
## Loading required package: permute
## Loading required package: lattice
## This is vegan 2.0-10
## Loading required package: nlme
library(phytools)
## Loading required package: maps
## Loading required package: rgl
## Warning: failed to assign RegisteredNativeSymbol for getData to getData
## since getData is already defined in the 'phangorn' namespace
We will use the same anolis data and phylogenetic tree from previous exercises. If you don’t already have them, you can download the files from the following addresses:
anolisDataAppended.csv
anolis.phy
If you need to, make sure these files are in your working directory and read them in.
anoleData <- read.csv("anolisDataAppended.csv", row.names = 1)
anoleTree <- read.tree("anolis.phy")
If you have the data, then the following commands should work:
plot(anoleTree)
name.check(anoleTree, anoleData)
## [1] "OK"
Let’s do the two main tests for phylogenetic signal using anole body size. The first test is Blomberg’s K, which compares the variance of PICs to what we would espect under a Brownian motion model. K = 1 means that relatives resemble one another as much as we should expect under BM; K < 1 means that there is less “phylogenetic signal” than expected under BM, while K > 1 means that there is more. A significant p-value returned from phylosignal tells you that there is significant phylogenetic signal - that is, close relatives are more similar than random pairs of species.
anoleSize <- anoleData[, 1]
names(anoleSize) <- rownames(anoleData)
phylosignal(anoleSize, anoleTree)
## K PIC.variance.obs PIC.variance.rnd.mean PIC.variance.P
## 1 1.554 0.1389 0.773 0.001
## PIC.variance.Z
## 1 -3.913
phylosig(anoleTree, anoleSize, method = "K", test = T)
## $K
## [1] 1.554
##
## $P
## [1] 0.001
Another method for testing phylogenetic signal is Pagel’s lambda. Lambda is a tree transformation that stretches tip branches relative to internal branches, making the tree more and more like a complete polytomy. If our estimated lambda = 0, then the traits are inferred to have no phylogenetic signal. Lambda = 1 corresponds to a Brownian motion model; 0 < lambda < 1 is in between.
# First let's look at what lambda does
anoleTreeLambda0 <- rescale(anoleTree, model = "lambda", 0)
anoleTreeLambda5 <- rescale(anoleTree, model = "lambda", 0.5)
par(mfcol = c(1, 3))
plot(anoleTree)
plot(anoleTreeLambda5)
plot(anoleTreeLambda0)
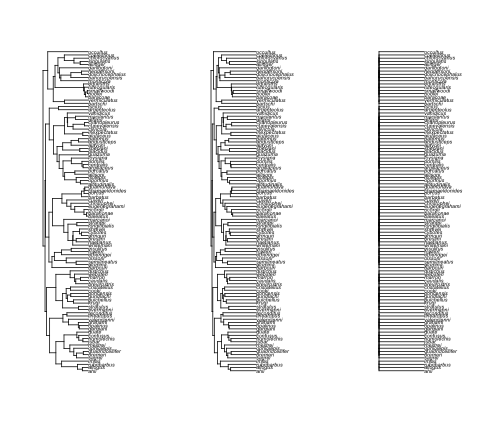
phylosig(anoleTree, anoleSize, method = "lambda", test = T)
## $lambda
## [1] 1.017
##
## $logL
## [1] -3.81
##
## $logL0
## [1] -60.02
##
## $P
## [1] 2.893e-26
lambdaModel <- fitContinuous(anoleTree, anoleSize, model = "lambda")
## Loading required package: parallel
## Warning: Parameter estimates appear at bounds:
## lambda
brownianModel <- fitContinuous(anoleTree, anoleSize)
nosigModel <- fitContinuous(anoleTreeLambda0, anoleSize)
lambdaModel$opt$aicc
## [1] 15.65
brownianModel$opt$aicc
## [1] 13.52
nosigModel$opt$aicc
## [1] 124.2
# Conclusion: Brownian model is best, no signal model is terrible
We can use fitContinuous to fit OU and EB models as well.
brownianModel <- fitContinuous(anoleTree, anoleSize)
OUModel <- fitContinuous(anoleTree, anoleSize, model = "OU")
EBModel <- fitContinuous(anoleTree, anoleSize, model = "EB")
# inspect results
brownianModel
## GEIGER-fitted comparative model of continuous data
## fitted 'BM' model parameters:
## sigsq = 0.136160
## z0 = 4.065918
##
## model summary:
## log-likelihood = -4.700404
## AIC = 13.400807
## AICc = 13.524519
## free parameters = 2
##
## Convergence diagnostics:
## optimization iterations = 100
## failed iterations = 0
## frequency of best fit = 1.00
##
## object summary:
## 'lik' -- likelihood function
## 'bnd' -- bounds for likelihood search
## 'res' -- optimization iteration summary
## 'opt' -- maximum likelihood parameter estimates
OUModel
## GEIGER-fitted comparative model of continuous data
## fitted 'OU' model parameters:
## alpha = 0.000000
## sigsq = 0.136160
## z0 = 4.065918
##
## model summary:
## log-likelihood = -4.700404
## AIC = 15.400807
## AICc = 15.650807
## free parameters = 3
##
## Convergence diagnostics:
## optimization iterations = 100
## failed iterations = 0
## frequency of best fit = 0.76
##
## object summary:
## 'lik' -- likelihood function
## 'bnd' -- bounds for likelihood search
## 'res' -- optimization iteration summary
## 'opt' -- maximum likelihood parameter estimates
EBModel
## GEIGER-fitted comparative model of continuous data
## fitted 'EB' model parameters:
## a = -0.736271
## sigsq = 0.233528
## z0 = 4.066519
##
## model summary:
## log-likelihood = -4.285970
## AIC = 14.571939
## AICc = 14.821939
## free parameters = 3
##
## Convergence diagnostics:
## optimization iterations = 100
## failed iterations = 0
## frequency of best fit = 0.41
##
## object summary:
## 'lik' -- likelihood function
## 'bnd' -- bounds for likelihood search
## 'res' -- optimization iteration summary
## 'opt' -- maximum likelihood parameter estimates
# calculate AIC weights
bmAICC <- brownianModel$opt$aicc
ouAICC <- OUModel$opt$aicc
ebAICC <- EBModel$opt$aicc
aicc <- c(bmAICC, ouAICC, ebAICC)
aiccD <- aicc - min(aicc)
aw <- exp(-0.5 * aiccD)
aiccW <- aw/sum(aw)
aiccW
## [1] 0.5353 0.1849 0.2798
It is important to realize that measurement error can bias your inferences with fitting these models towards OU. Fortunately, we can easily account for that in fitContinuous.
# We measured 20 anoles per species, and the standard deviation within each
# species was, on average, 0.05
seSize <- 0.05/sqrt(20)
# redo with measurement error
brownianModel <- fitContinuous(anoleTree, anoleSize, SE = seSize)
OUModel <- fitContinuous(anoleTree, anoleSize, model = "OU", SE = seSize)
EBModel <- fitContinuous(anoleTree, anoleSize, model = "EB", SE = seSize)
# calculate AIC weights
bmAICC <- brownianModel$opt$aicc
ouAICC <- OUModel$opt$aicc
ebAICC <- EBModel$opt$aicc
aicc <- c(bmAICC, ouAICC, ebAICC)
aiccD <- aicc - min(aicc)
aw <- exp(-0.5 * aiccD)
aiccW <- aw/sum(aw)
aiccW
## [1] 0.5346 0.1846 0.2808